Breadcrumb
- Home
- Research
Research
Pesticide exposure during neurodevelopment
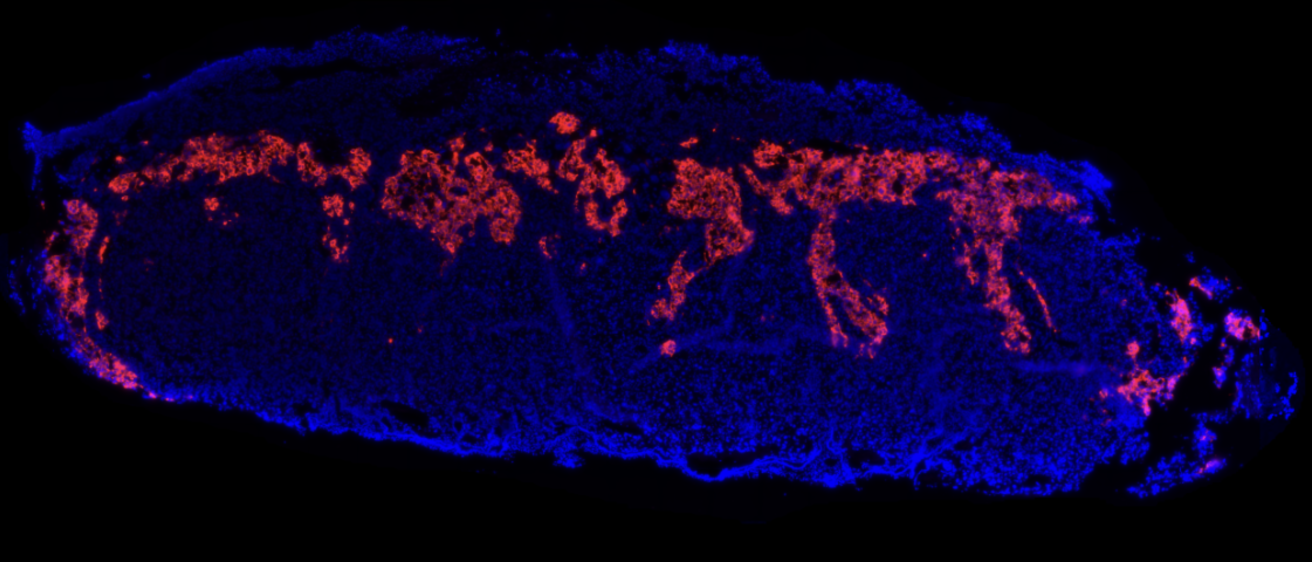
Adolescent brain development is sensitive to exposures and has impacts on neurobehavioral functioning. Insecticide exposure may impact executive and attentional brain circuits which mature during adolescence due to pubertal hormonal influences, and these changes may manifest as ADHD-related symptoms. Through a project in collaboration with other investigators at University of Iowa, the University of Buffalo, and Rutgers University, we are expanding our knowledge on how insecticide exposure affects the adolescent brain. Our research is focused on the effects of α-cypermethrin and chlorpyrifos, informed by our collaborative group's extensive research in the occupational exposure of these pesticides in a cohort of Egyptian adolescent pesticide-applicators. Our lab is utilizing a mouse model to investigate behavioral effects of these toxin exposures as well as the underlying mechanisms responsible for these effects, with special interest in oxidative stress as a mechanism, the role of dopaminergic neurons in this process, and testosterone as a moderator of these effects.
The Influence of Placental IGF-1 Expression on Fetal Striatal Development
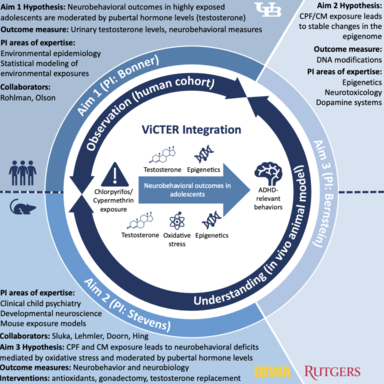
The placenta is essential in fetal neurodevelopment for vital growth factors and is the primary source of Insulin-like growth factor 1 (IGF1) to the fetus. IGF1 is necessary for mediating fetal growth, especially in the striatum, and is a major regulator of placental nutrient transporters. Cross-sectional studies have implicated a connection between ASD, ADHD, and IGF1, but the pathophysiological role of IGF1 is yet to be defined. IGF1 is required for neurodevelopment, the striatum highly expresses IGF1R, and the striatum controls procedural, habit, and reversal learning. Striatal structure and function are altered in some with ASD and ADHD. Determining the effect of placental biology on fetal neurodevelopment is essential to understand the complex origin of NDDs. We aim to test this by manipulating placental expression of IGF1 in mice. We can knockout and overexpress IGF1 via a placental targeted CRISPR manipulation developed in the lab. Additionally, we have a placenta specific Cre mouse line as another method to knockout placental IGF1. Embryonic timepoints are analyzed to evaluate changes to placental function and embryonic brain development. We also aim to characterize long-term impacts of placental IGF1 expression changes through animal behavior tests and brain analyses with a focus on striatal-dependent changes.
Effects of Prenatal Stress on the Striatum
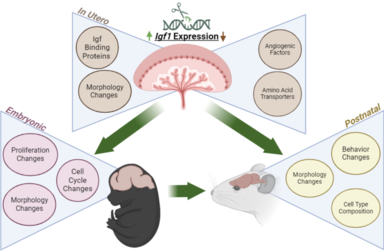
Prenatal stress, or maternal stress during pregnancy, has effects on the developing offspring brain. We are specifically interested in its effects on the dorsal striatum, which is relevant to neurodevelopmental disorders such as autism spectrum disorder (ASD). We study the effects of prenatal stress on striatal-dependent behaviors relevant to ASD using a mouse model (Aim 1.1). Additionally, we study cellular and molecular outcomes in the striatum with techniques such as immunohistochemistry and single-cell RNA sequencing to examine specific cell populations including D1 and D2 medium spiny neurons (MSNs) (Aim 1.2). Finally, we induce overgrowth of the embryonic striatum via intracerebroventricular injection of CHPG and test how this affects striatal-dependent behaviors in mice (Aim 2).
Find Us at Conferences

- Society for Neuroscience (SfN)
- US Developmental Origins of Health and Disease (DOHaD)
- American College of Neuropsychopharmacology (ACNP)
- Society for Developmental Biology (SDB)
- The Allied Genetics Conference (TAGC)
- American Society for Biochemistry and Molecular Biology (ASBMB)
- Wisconsin Symposium on Emotion (WSoE)
Funding Sources





